What you need to know
Lime can be a powerful tool to combat acidic soils. Here’s what farmers in Minnesota need to know:
-
Agricultural liming materials include limestone (both calcitic and dolomitic), burned lime, slaked lime, marl, shells, and by-products like sugar beet lime and sludge from water treatment plants.
-
In Minnesota, liming materials are analyzed and sold on the basis of Effective Neutralizing Power (ENP).
-
Select a lime material that allows for uniform application across a field.
What is a liming material?
An agricultural liming material contains calcium (Ca) and/or magnesium (Mg) compounds capable of neutralizing soil acidity. These materials include: limestone (both calcitic and dolomitic), burned lime, slaked lime, marl, shells, and by-products like sugar beet lime and sludge from water treatment plants.
Fluid lime describes the concept of suspending liming materials of various types in either water or fertilizer solutions. That liming material is usually finely ground agricultural limestone with a high neutralizing value. Advantages include rapid availability and application with existing fluid fertilizer equipment. Drawbacks are low rates of application and relatively high cost for the lime applied.
Lime neutralizes soil acidity
Everything that contains calcium or magnesium is not necessarily a liming material. Gypsum, for example, is calcium sulfate (CaSO4 • H2O). When added to the soil, the calcium in the gypsum can displace the hydrogen on a clay particle. The hydrogen, however, would remain in the soil solution and the pH would not change because of the absence of carbonate.
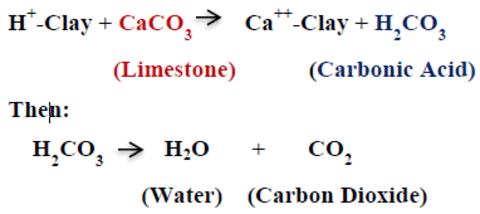
When added to the soil, calcium and/or magnesium dissolved from the liming materials displace hydrogen (H+) from the clay particles. It is the hydrogen ion (H+) that makes soils acid. The displaced hydrogen then reacts with carbonate, reducing soil acidity. Carbonate dissolved from the limestone materials forms carbonic acid. Carbonic acid is not stable in soils and quickly forms carbon dioxide and water. With this chemical process, the hydrogen (H+) has been converted from an ion on a clay particle to a neutral molecule of water, thereby reducing soil acidity. The chemical reaction for this process is shown at the top of the page.
Limestone quality
In Minnesota, liming materials are analyzed and sold on the basis of Effective Neutralizing Power (ENP). The analysis label lists pounds of ENP per ton of liming material. Similarly, lime suggestions are now provided in terms of lb of ENP per acre. The ENP is calculated from an analysis of carbonate content and a measure of particle sizes. In the past, recommendations were made in terms of tons per acre. View specific suggestions of pounds ENP per acre for major crops in Minnesota.
The chemical purity of the limestone material is expressed on the basis of Calcium Carbonate Equivalent (CCE) on a dry weight basis. Moisture content must be determined for all liming materials to determine percent dry matter, which is required for calculating material application rate. The Fineness Index (FI) is determined in the laboratory by measuring the percentage of the liming material that passes through sieves of various sizes. Three sieve sizes (8 mesh, 20 mesh, 60 mesh) are used. The FI is determined from the following equation:
FI = ( % passing 8 mesh but remaining on 20 mesh) x0.2
+ ( % passing 20 mesh but remaining on 60 mesh) x 0.6
+ ( % passing 60 mesh) x 1.0
The smaller particles (those that pass through the 60 mesh sieve) bring about a rapid change in soil pH. The larger particles (those that pass through the 8 mesh sieve but remain on the 20 mesh sieve) dissolve more slowly in soils and provide for an increase in pH over a longer period of time.
In general, ag lime is a mixture of particles of various sizes. This mixture provides for both a rapid increase in soil pH and maintenance of this increase for a period of time.
The percent ENP is calculated from the following equation:
% ENP = % CCE X FI ÷ 100
Once % ENP is determined, the moisture content (% dry matter) of the material is needed to calculate the minimum pounds of ENP per ton of liming material as follows:
(2000 lbs/ton X % ENP ÷ 100) X (% dry matter ÷ 100)
A variety of liming materials
For several years, ground agricultural limestone or ag lime was the primary liming material used in Minnesota. There are, however, a number of by-product materials that can be used to increase soil pH. In some situations, these materials are given to the grower. In other cases, there is no or a small charge for the material, but the grower pays the cost of hauling. The moisture content and TNP of these materials varies over a wide range. The grower who uses these products should have them analyzed. With this analysis, it's possible to determine the pounds of ENP per acre to apply to bring the soil pH to a desired level.
There is one other factor to consider when choosing a liming material. The liming material selected should spread so that it's possible to achieve a uniform application over the entire field. Ag lime spreads easily. On the other hand, liming material that contain relatively large amount of water (sugar beet lime (PCC); water softening lime) are more difficult to spread uniformly over the field. This lack of uniform spreading could cause production problems for several years after application.
If applied to supply equivalent amounts of ENP per acre, all liming materials should have an equal effect on crop yield. So, the decision on source to use should be based primarily on cost.
Dolomitic versus calcitic limestone
Most of the Ag lime quarried in Minnesota contains both calcium and magnesium. Both of these nutrients are essential for crop production. Calcium requirements of crops are low and Minnesota soils contain ample amounts of this nutrient. There are some who believe that, when lime is needed, only calcitic lime should be used.
This belief originates from a concept which suggests that there is an ideal ratio of calcium to magnesium in soils and any deviation from this ratio will cause problems with crop production. Several field trials have been conducted to test the validity of this concept. The results are clear: the ratio of calcium to magnesium in soils has not had any effect on crop yield in the northern Com Belt. Wisconsin researchers, for example, varied the ratio of calcium to magnesium from 2 to 8 and found no effect on the yield of alfalfa grown on a sandy soil and a silt loam soil.
The calcium to magnesium ratio is not important in Minnesota soils. However, the supply of magnesium can affect production. Magnesium will be needed in a fertilizer program if the soil test for magnesium is low. The use of dolomitic lime is one of the easiest and most cost effective ways to add magnesium to soils.
Economic considerations
The determination of the ENP of various liming materials has economic implications for the grower. With this measurement, it's now possible to compare various liming materials on a cost basis. The following equation can be used to calculate this cost:
Cost/lb ENP = (Price/ton of Material) / (lb of ENP per ton)
The Lime Law and the lime user
The implementation of the Minnesota Lime Law in 1990 was a major step forward in allowing for a true comparison among lime sources. Although new terminology was introduced in making liming suggestions, it should not be confusing. This law does provide a standardized system for calculating costs. Growers have a basis for computing costs of a wide variety of liming materials. This ability to calculate costs and choose liming materials based on costs improves the profitability of Minnesota growers who need lime for crop production.
Reviewed in 2023